Beware of Galvanic Action (It's a Thing)


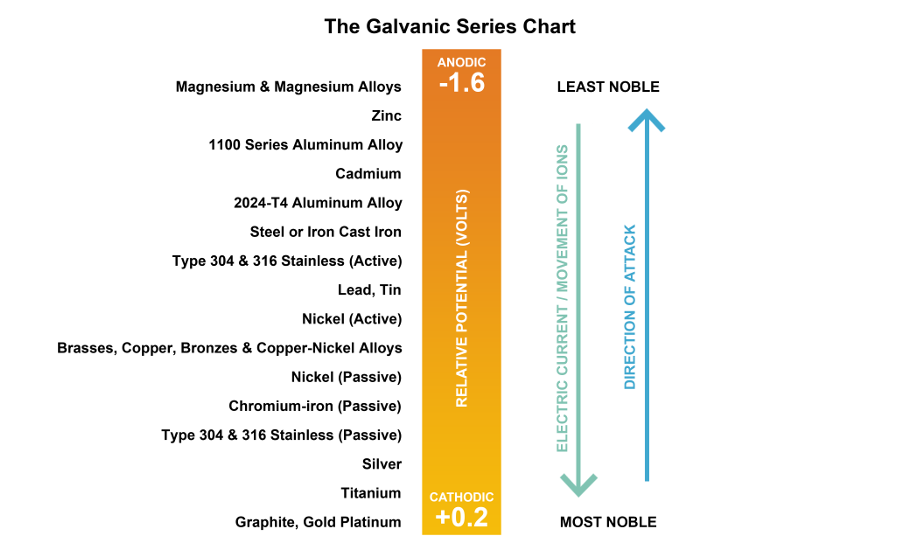
Figure 1: The Galvanic Series Chart. The order of the metals are general and meant to serve for referential purposes here. In application, these metals can exhibit a range of voltage potential relative to a reference electrode. This series of alloys assumes full submersion in flowing seawater. Figure by Daniel Overbey.

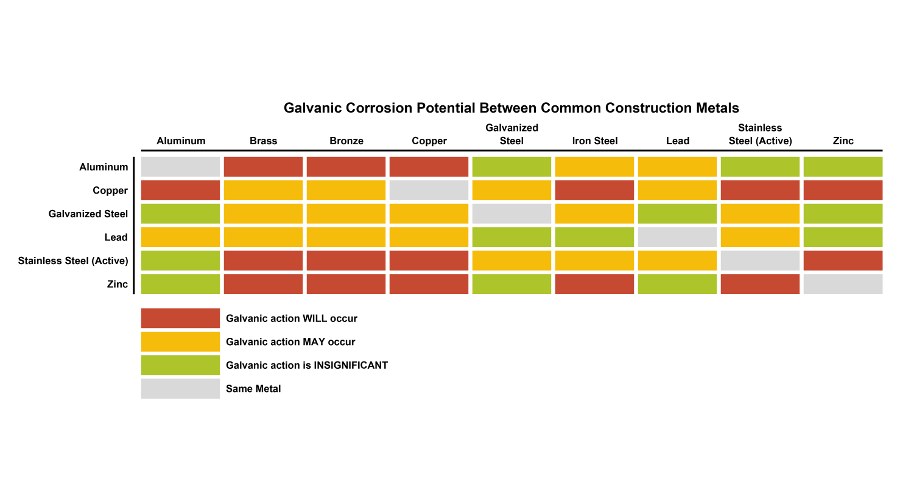
Figure 2: Galvanic Corrosion Potential Between Common Construction Metals. Illustration by Daniel Overbey. Adapted from Stuart, 2013.



The concept of "galvanic action" is confounding to many design professionals. In simple terms, galvanic action refers to the corrosive effect created when two electrochemically dissimilar metals are in direct contact with each other. Their contact creates a conductive path for electrons and ions to move from one metal to the other. As ions move from one metal to another, corrosion occurs. The remedy is to keep these dissimilar metals insulated from each other to drastically slow the corrosive effect.
We know it is unwise to play in an outdoor swimming pool when lightning is present. Generally, water creates a conductive path - especially salt water. Therefore, it is critical to keep dissimilar metals separated in wet conditions.
The Galvanic Series Chart
Cathodes are noble - or stable - metals, meaning they are less prone to corrosion. Historically, silver and gold have been preferred for jewelry and currency because they are less susceptible to gradually destruct while in one's possession.
Anodes are less stable and more prone to corrosion. Zinc and aluminum a common metals with higher anodic properties.
The chart below exhibits several common metals and where their relative location in the galvanic series (Fig. 1). When two metals close in this chart are in contact, there is less galvanic action (corrosion of the more anodic metal). Conversely, if the two metals in contact are farther away on the chart, galvanic action is more likely to occur.
Galvanic action can occur when improper fasteners are used in a building facade. Image a stainless steel building component (fairly cathodic) is fastened in place using galvanized (zinc-coated; highly anodic) metal fasteners. The galvanized fasteners would corrode as the ions in the fasteners moved toward the stainless steel. Eventually, the corroded fasteners would fail - leading to damage and possible injury.
A classic example of galvanic corrosion is the Statue of Liberty. I recently had the chance to visit the Statue of Liberty Museum in New York and learned that the monument underwent a major renovation in the 1980s. Among other issues addressed, over time galvanic corrosion of the wrought iron armatures - a key component of Lady Liberty's superstructure - was threatening to compromise the entire monument. The metal had to be replaced - a costly and time-consuming undertaking. However, a decade after the restoration, an inspection noted that the corrosion problems had been successfully corrected.
The chart below may serve as a quick reference regarding galvanic corrosion potential between common construction metals (Fig. 2). It is important to note that any alloy may exhibit a range of voltage potential relative to a reference electrode. Specifications should be carefully coordinated and details should be designed to ensure that dissimilar do not have contact - either directly or via submersion in a highly-conductive fluid medium.
Reference:
Stuart, D. Matthew, 2013, Dissimilar Metal Corrosion. PDH Online Course, Fairfax, VA.